
Curriculum Vitae
- B.A., chemistry and biochemistry, Rice University, 1989
- Ph.D., medicinal chemistry, Purdue University, 1998
Research Interests
I. Ergolines
Ergot alkaloids exhibit a vast array of pharmacological activity, perhaps more so than any other class of natural products. They are an ideal chemical starting point from which to produce a range of libraries as probes of biological targets. Foremost among these targets are monoamine brain receptors, such as the serotonin receptors 5-HT5A, 5-HT6, and 5-HT7, for which few selective ligands exist. The diversity of bioactivity found in naturally occurring ergolines, ranging from neuroreceptor agonism and antagonism to tyrosine kinase inhibition, suggests that constructing a synthetic library of analogous structures would yield a large number of active compounds. The fact that small changes in the structures of ergolines can produce dramatic differences in activity means that such libraries are likely to exhibit a rich pharmacology.
The aim of our current research is to construct ergoline libraries with diverse substituents on the A (phenyl) ring. We have synthesized a complex pyridine intermediate that allows rapid synthesis of variants of the ergoline nucleus from phenylhydrazines in only two steps. As an added bonus, the method provides a way to tune the natural fluorescence wavelength of ergolines to yield a set of biological probes with inherent self-reporting fluorescence properties.
II. HCV inhibitors
The hepatitis C virus (HCV), having infected some 200 million people worldwide, is a major threat to human health. Currently available interferon-based treatments are associated with serious side effects and are effective only in certain patients.
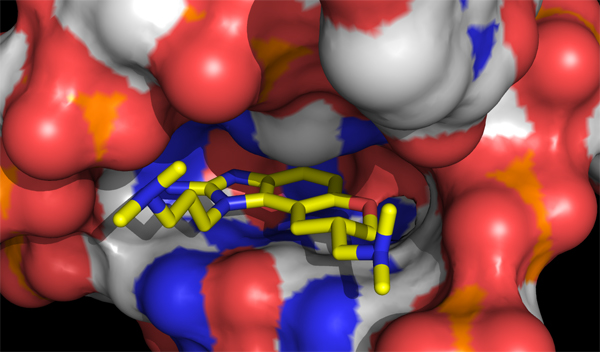 Although HCV protease inhibitors have recently become available as a new line of therapy, the existence of drug-resistant strains confirms the continued need for anti-HCV drugs. Recently a class of small-molecule drugs was discovered that acts against HCV in a new way, binding to a region of the viral RNA required for initiation of protein synthesis (the IRES-IIa subdomain).
In early 2011 we reported a streamlined route to this class of inhibitors. A key innovation is the attachment of a variable side chain at a late point in the synthetic route.only three steps from the end.providing an opportunity for a divergent synthesis of new HCV drug candidates.
Although HCV protease inhibitors have recently become available as a new line of therapy, the existence of drug-resistant strains confirms the continued need for anti-HCV drugs. Recently a class of small-molecule drugs was discovered that acts against HCV in a new way, binding to a region of the viral RNA required for initiation of protein synthesis (the IRES-IIa subdomain).
In early 2011 we reported a streamlined route to this class of inhibitors. A key innovation is the attachment of a variable side chain at a late point in the synthetic route.only three steps from the end.providing an opportunity for a divergent synthesis of new HCV drug candidates.
Improved access to the lead compound has enabled our collaborator, T. Hermann at UCSD, to crystallize it bound to the IRES-IIa subdomain and obtain an x-ray diffraction structure. We are now creating synthetic routes to new analogs, using this crystallographic data as a guide, in a search for more potent and selective compounds.
Selected Publications
- Dibrov, S.M.; Ding, K.; Brunn, N.D.; Parker, M.A.; Bergdahl, B.M.; Wyles, D.L.; Hermann, T. Structure of a hepatitis C virus RNA domain in complex with a translation inhibitor reveals a binding mode reminiscent of riboswitches. Proc. Natl. Acad. Sci. USA 2012, 109, 5223-5228.
- Parker, M. A.; Satkiewicz, E.; Hermann, T.; Bergdahl, B. M. An Efficient New Route to Dihydrobenzimidazole Inhibitors of HCV Replication. Molecules 2011, 16, 281-290.
- Sebastiani, D.; Parker, M. A. Polyhedral Phenylacetylenes: The Interplay of Aromaticity and Antiaromaticity in Convex Graphyne Substructures. Symmetry 2009, 1, 226-239.
- Lakner, F. J.; Parker, M. A.; Rogovoy, B.; Khvat, A.; Ivachtchenko, A. Synthesis of Novel Trisubstituted Imidazolines. Synthesis 2009, 1987-1990.
- Parker, M. A.; Kurrasch-Orbaugh, D. M.; and Nichols, D. E. The Role of Lipophilicity in Determining Binding Affinity and Functional Activity for 5-HT2A Receptor Ligands. Bioorg. Med. Chem. 2008, 16, 4661 4669.
- Bologa, C. G.; Revankar, C. M.; Young, S. M.; Edwards, B. S.; Arterburn, J. B.; Kiselyov, A. S.; Parker, M. A.; Tkachenko, S. E.; Savchuk, N. P.; Sklar, L. A.; Oprea, T. I.; and Prossnitz, E. R. Virtual and Biomolecular Screening Converge on a Selective Agonist for GPR30. Nature Chemical Biology 2006, 2, 207-212.
