Tom Huxford
Professor, Biochemistry
Department Chair
office: CSL 325A
phone: 619-594-1606
email: thuxford@sdsu.edu

Huxford Group Page
Curriculum Vitae
- B.S. Biochemistry, Brigham Young University, Provo, Utah, 1995
- Ph.D. Chemistry, University of California San Diego, La Jolla, California, 2001, Advisor: Dr. Gourisankar Ghosh
- Postdoc Structural Biology, University of California San Diego, La Jolla, California, 2005, Fellow of the University of California University-wide AIDS Research Program
- Assistant and Associate Professor, Department of Chemistry & Biochemistry, San Diego State University, 2005-2013
- Assistant and Associate Professor, Molecular Biology Institute, San Diego State University, 2005-2013
- Professor, Department of Chemistry & Biochemistry, San Diego State University, 2013-present
- Professor, Molecular Biology Institute, San Diego State University, 2013-present
- Chair, Department of Chemistry & Biochemistry, San Diego State University, 2024-present
Research Interests
In this laboratory we use our knowledge and experience in the area of protein structure and function to determine the chemical mechanisms employed by interesting biological factors. The major focus of the laboratory is in understanding regulation in the transcription factor NF-κB signal transduction pathway. NF-κB is a relatively small class of proteins that respond to diverse stimuli by activating the expression of numerous genes. NF-κB responsive genes include many of the key components of the cellular survival program including inflammatory cytokines, mediators and effectors of both innate and adaptive immunity, and inhibitors of apoptosis. Although proper NF-κB function is integral to a cell's ability to fight off infection and respond to stress, too much of an NF-κB response can contribute to states of chronic inflammation such as arthritis, asthma, multiple sclerosis, and colitis. Recently, it has been shown that chronically inflamed tissues can serve as hotbeds for tumor formation. Cellular processes that recognize and kill tumors in healthy tissues fail to function effectively under the influence of the NF-κB cell survival program. Chronic inflammation due to hyperactive NF-κB has also been shown to contribute to sclerotic formation in arteries and heart disease.
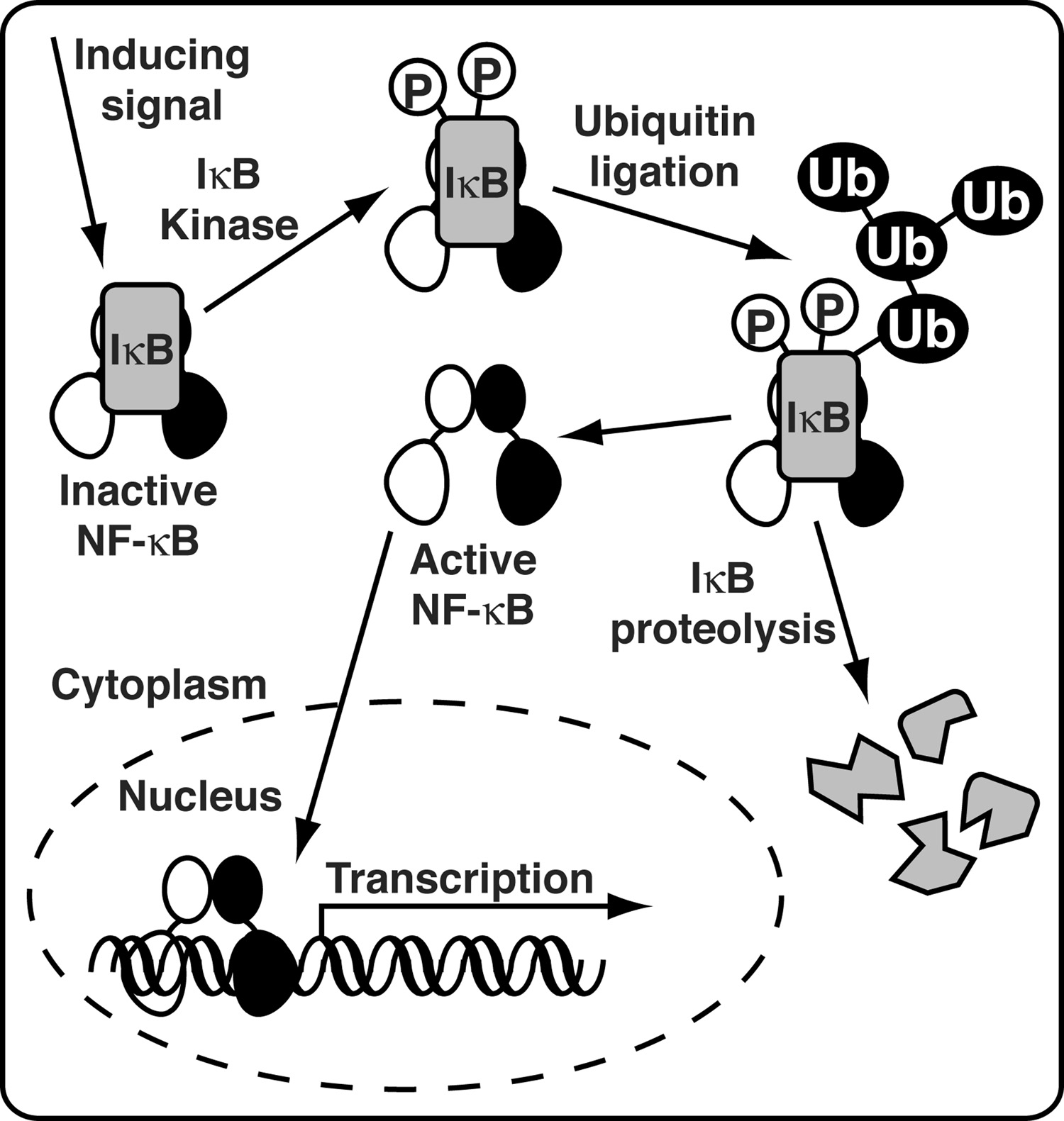
The prototypical NF-κB functions as a heterodimer of p50 and p65 subunits. NF-κB is present in the cytoplasm of all cells as an inactive factor in complex with a member of the IκB inhibitor protein family. Diverse NF-κB-inducing stimuli lead to activation of the IκB kinase complex (IKK). IKK is a large multisubunit complex that specifically phosphorylates a pair of serine amino acid side chains in the amino-terminal region of NF-κB complex-associated IκB. Once phosphorylated, IκB is recognized by a specific E3 Ubiquitin-protein ligase complex leading to its poly-ubiquitinylation. The 26 S proteasome can then recognize and proteolyze IκB. Removal of IκB renders NF-κB active. It rapidly translocates from the cytoplasm to the nucleus where it binds specifically to DNA elements within the promoter regions of target genes and activates their transcription (Figure 1).
We are currently working on the following two NF-κB-related projects:
-
IKK structure and function.
IKK is a multisubunit kinase complex that specifically phosphorylates IκB. Purification of IKK from cytokine-induced HeLa cells revealed that it is composed of three subunits. These are referred to as IKKα (IKK1), IKKβ (IKK2), and IKKgamma (NEMO, FIP3). Although IKKα and IKKβ are highly conserved protein subunits, they differ significantly in their cellular function. For example, the IKKβ subunit has been shown to be responsible for activating NF-κB in response to inflammatory stimuli by catalyzing the attachment of two phosphates near the amino-terminus of the classical IκB proteins. Furthermore, IKKβ itself is subject to phosphorylation-dependent regulation of its own catalytic activity. We are interested in understanding the detailed mechanisms of substrate specificity and phosphorylation-dependent regulation of the IKKβ subunit. We are also interested in understanding the structure of multisubunit IKK complexes including the role of IKKgamma in regulating catalytic activity of the enzyme.
-
Nuclear IκB structure and function.
The classical NF-κB inhibitor proteins, IκBα, IκBβ, and IκBε, function primarily in the cell cytoplasm by masking NF-κB nuclear localization signals and blocking DNA binding. However, two additional classes of IκB proteins are also integral to NF-κB regulation. The proteins p105 and p100 play a dual roles as IκB proteins and precursors of the mature NF-κB p50 and p52 subunits, respectively. The identification of a third general class of IκB proteins that function exclusively in the nucleus has been made recently. The nuclear IκB proteins include Bcl-3, IκBζ (MAIL), and IκBNS. These proteins all show similar properties: their expression is regulated by NF-κB; they rapidly accumulate in the nucleus; and they have modulatory effects on NF-κB-dependent expression of specific target genes. We have shown that in contrast to classical IκB proteins, the nuclear IκBζ protein binds preferentially to the NF-κB p50 homodimer. We also found that formation of this protein-protein complex does not remove the NF-κB homodimer from binding to target DNA. We are currently interested in studying how assembly of an IκBζNF-κB p50:DNA complex in the nucleus activates the expression of specific NF-κB responsive genes such as the cytokine interleukin-6 (IL-6).
Other projects in which we are currently involved include:
-
Structure and function of a muscle assembly co-chaperone
In collaboration with the students in the laboratory of Dr. Sanford I. Bernstein in the Department of Biology at San Diego State University we are studying the structure and in vitro biochemistry of the factor UNC-45 that functions to help assemble skeletal muscles and to repair the misfolded heads of myosin motor proteins. We are also using a novel method for recombinant expression and purification of myosin isoforms from whole flies to study the structural and biophysical consequences of disease-causing mutations on motor proteins.
-
Structure and antigen binding of therapeutic antibodies
We collaborate with several local biotechnology and pharmaceutical companies to understand the structural mechanisms of clinically relevant antibodies. Current projects in this area include: selective antigen binding of anti-lipid antibodies, antibodies that employ metal ions as bridging factors in antigen binding, and novel bispecific antibody designs to specifically target difficult antigens.
- "Structural and biochemical analyses of the nuclear IκBζ protein in complex with NF-κB p50 homodimer,"
Zhu N., Rogers W.E., Heidary D.K., Huxford T.,
Genes & Development 38, 528-535 (2024). (doi: 10.1101/gad.351892.124.) - "Aging-affiliated post-translational modifications of skeletal muscle myosin affect biochemical properties, myofibril structure, muscle function, and proteostasis,"
Neal C., Kronert W.A., Camillo J.R.T, Suggs J.A., Huxford T., Bernstein S.I.,
Aging Cell 23, e14134 (2024). (doi: 10.1111/acel.14134.) - "Active site remodeling in tumor-relevant IDH1 mutants drives distinct kinetic features and potential resistance mechanisms,"
Mealka M., Sierra N.A., Avellaneda Matteo D., Albekioni E., Khoury R., Mai T., Conley B.M., Coleman N.J., Sabo K.A., Komives E.A., Bobkov A.A., Cooksy A.L., Silletti S., Schiffer J.M., Huxford T., Sohl C.D.,
Nature Communications 15, 3785 (2024). (doi: 10.1038/s41467-024-48277-2.) - "X-ray crystallographic study of preferred spacing by the NF-κB p50 homodimer on κB DNA,"
Zhu N., Mealka M., Mitchel S., Milani C., Acuña L.N., Rogers E., Lahana A.M., Huxford T.,
Biomolecules 13, 1310 (2023). (doi: 10.3390/biom13091310.) - "TWEAK-Fn14-RelB Signaling Cascade Promotes Stem Cell-like Features that Contribute to Post-Chemotherapy Ovarian Cancer Relapse,"
Holmberg R., Robinson M., Gilbert S.F., Lujano-Olazaba O., Waters J.A., Kogan E., Velasquez C.L.R., Stevenson D., Cruz L.S., Alexander L.J., Lara J., Mu E.M., Camillo J.R., Bitler B.G., Huxford T., House C.D.,
Molecular Cancer Research 21, 170-186 (2023). (doi: 10.1158/1541-7786.MCR-22-0486.) - Myung Soo Ko, Ph.D. Dissertation 2020. Understanding the role of NEMO in IKK activation.
- Samantha Cohen, Ph.D. Dissertation 2021. Structural and biochemical characterization of the Drosophila IKK complex: a key component of the IMD innate immune signaling pathway in flies.
- Shane Mitchel, M.S. Thesis 2023. DNA binding and structure of a basal metazoan homolog of transcription factor NF-κB.
- Ryne Holmberg, Ph.D. Dissertation 2023. TWEAK-Fn14-RelB signaling cascade promotes stem cell-like features that contribute to post-chemotherapy ovarian cancer relapse.
- Jorge Rodriguez, M.S. Thesis 2024. Investigation into the protein-protein interactions of UNC-45.
